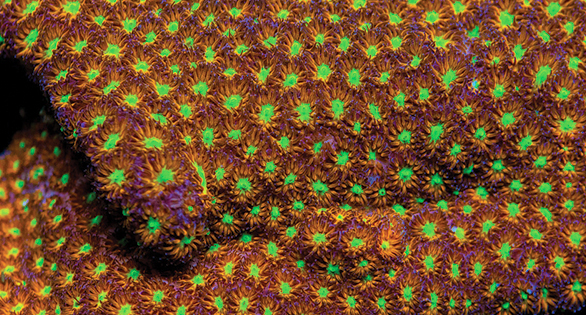
Jason Fox “Spit Fire” Leptastrea: color morphs such as this may present an opportunity to stony coral propagators. Image: May Fox.
By Samuel Nietzer,
Institute for Chemistry and Biology of the Marine Environment
University of Oldenburg
Excerpt from CORAL, March/April 2019
In the wild, Leptastrea purpurea, with which a few scientists have been working for many years, is typically rather plain-colored and a bit of an outcast—formerly a member of the family Faviidae. In the current taxonomic upheaval triggered by DNA profiling, the genus Leptastrea is an orphan, not assigned to any family (“incertae sedis” according to Budd et al., 2012). Although very attractive colonies with hues of gold, purple, vivid green, and orange are seen in the U.S. aquarium trade, the Guam population I know best is mostly brown to yellowish or ocher-colored. Still, it is an amazing coral and well worth knowing.
Distribution

Author Samuel Nietzer on a reef in Guam.
Leptastrea purpurea has an extensive distribution and can be found from the reefs of the western Indian Ocean to the Indo-Pacific Coral Triangle to the Hawaiian Islands. The species is also common and found in large numbers on many reefs in Guam, especially on fringing reefs such as the Luminao Reef on the west coast of Guam, where it grows mainly in shallow depths from just below the surface to about 6 feet (2 m). But in Guam, L. purpurea is found not only in zones of strong tidal influence but also commonly in depths of 130 feet (40 m) or more. The large variability of the habitats indicates that L. purpurea possesses an unusual degree of adaptability and a generalist way of life.
In particular, its occurrence in extreme habitats such as the shallow fringing reef at Luminao, where the water temperature can easily reach 93°F (34°C) at a midday low tide, marks L. purpurea as a pioneer species. The most extreme habitat in which we have encountered L. purpurea in Guam is in tide pools that are usually connected to the ocean only at high tide and through the porous limestone. These natural seawater basins at Inarajan on the southeastern coast of Guam are a very popular destination for locals and tourists alike. At midday, the pools can heat up to 95°F (35°C). Murky with sediment and algae, they initially appear to be extremely coral-hostile biotopes. Because of this, it is all the more surprising to find large specimens of L. purpurea encrusting the rock and thriving.

Typical wild-collected Leptastrea purpurea. Although often somewhat drab in coloration, more eyecatching morphs are making their way into the aquarium trade and may offer interesting opportunities for captive breeding efforts.
Reproductive strategies
The most astonishing and unique feature of L. purpurea is not, however, its apparent adaptability but its reproductive strategy. Stony corals have two different strategies for sexual reproduction. By far the most common is seasonal mass spawning, in which all the corals of a species simultaneously release their reproductive cells into the surrounding seawater. The synchronization of the onset of this spawning, sometimes precise down to the minute, is determined mainly by the lunar cycle and day length. In these species, fertilization, as well as embryonic development, take place in the water column. Some 80 percent of known species of stony coral reproduce in this way.
The second reproductive strategy is so-called brooding, in which parent colonies harbor their growing progeny before releasing them. First, sperm are released into the water and are taken in by other Leptastrea colonies. Fertilization occurs in the interior of the polyp, which is also where planula larvae develop. After a specific period of development, the fully-formed larvae are released into the water column.
However, there are also species (for example, Pocillopora damicornis) that can produce planula larvae not only by normal fertilization but also by cloning, i.e., in a parthenogenetic manner. In the majority of known brooding species, reproduction occurs at certain times of year or in cycles. Leptastrea purpurea colonies in Guam were found to have both male and female genetic traits in their polyps, and the species thus reproduces sexually by hermaphroditic brooding.
The extraordinary thing about the reproduction isn’t, however, the strategy, but the daily release of larvae. This circumstance makes L. purpurea a particularly suitable species for performing experiments with planula larvae. In all the years we have been working with L. purpurea, we have been unable to determine whether larvae are released in a cycle or if reproduction takes place seasonally.
In particular, the settlement behavior of the coral larvae is a major area of research in our laboratory, in which the chemical triggers of larval settlement are being identified. We have observed that larvae of L. purpurea show a very similar settlement behavior to larvae of ecologically important Acropora species and react to the same chemical stimuli, and this makes the very hardy Leptastrea an extremely suitable subject for study.
Collecting corals
In order to obtain larvae of Leptastrea, you need a certain number of adult colonies as breeding stock. In Guam, we collected these mainly on the Luminao Reef (with the permission of the local authority for aquatic resources). At the Marine Biology Institute of the University of Guam, the broodstock corals are placed in large through-flow tanks where they can recover from the stress of collection and transportation for several days. Scratches and other superficial injuries are usually covered by living tissue after a few days.
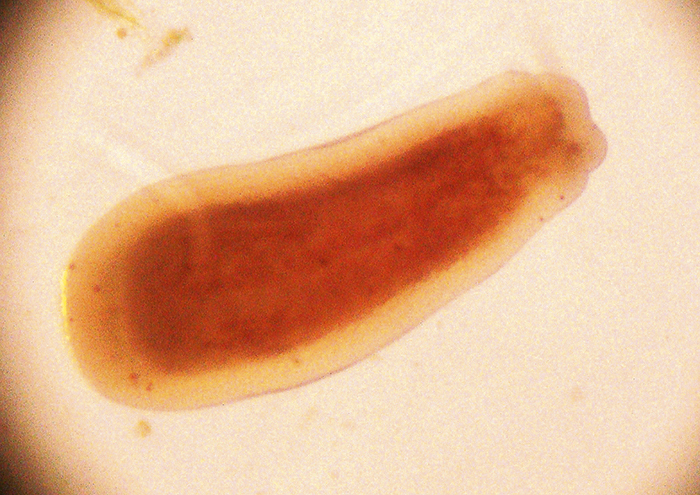
Leptastrea purpurea larva, image greatly enlarged.
After the acclimatization period, we usually place the corals on lighting-grid “eggcrate” panels measuring around 12 x 16 inches (30 x 40 cm). Suspended on cable ties, these panels, which collect larvae, fit into containers with a volume of about 8 gallons (30 L). In the evening, when spawning occurs, the corals, on their grids, are transferred to the collection containers. The grid panels are ideal: they make it possible to transport the corals without anyone touching them, and the corals stay in the same position which keeps stress as low as possible.
Harvesting larvae
Provided with adequate aeration, the corals spend the night in the containers. Because these containers aren’t operated as part of a through-flow system, the corals can readily be fed with Artemia nauplii during the night. The next morning, the corals are carefully transferred back to the through-flow tanks. The water from the containers is filtered through a fine sieve and the material obtained is transferred to a glass bowl. The larvae can be separated from this material, which consists mainly of the digestive products of the coral polyps, fine sediment, algae particles, and other organic material.
A particularly fortunate factor we had not expected is the presence of green fluorescent protein (GFP) in the larvae. The precise cause of the high levels of GFP in the larvae has not yet been explained, but protection against light may play a role. If you shine blue light on GFP, it is reflected back as green light as a result of the Stokes shift. The excess blue light can be eliminated using yellow-filter glasses so that you see mainly the fluorescent surfaces, and the larvae are clearly and unmistakably visible.
Based on our experiences to date, a coral measuring about 1.5–3.0 inches (4–8 cm) in diameter releases about four larvae per night, so around 1,000 larvae can be obtained every day from our lab’s broodstock of 250 corals. However, the number of larvae released decreases the longer collection continues. We assume that despite all precautionary measures the stress to which the corals are exposed plays an important role here. Also, failure to ingest sufficient food in captivity could be contributing to the decrease in larvae released.
Normally, L. purpurea corals begin catching prey with the onset of darkness. If you don’t feed them enough during the night, this may influence the energy-consuming production of larvae. In addition, with the passage of time, nuisance organisms such as Aiptasia, hydroids, and benthic ctenophores can be found on the grid between the corals and may harm them. If, however, you give the corals a few weeks to recover and provide them with sufficient high-quality planktonic food, the number of larvae released increases again.
There is a one-day peak of over 2,000 larvae in the diagram, which shows the number of larvae collected daily over a period of 65 days. We suspect that this unusual number can be attributed to heat stress, as unusually high temperatures were recorded in Guam on that day. Despite the through-flow system, in such cases, it is difficult to avoid the holding tanks heating up by a few degrees.
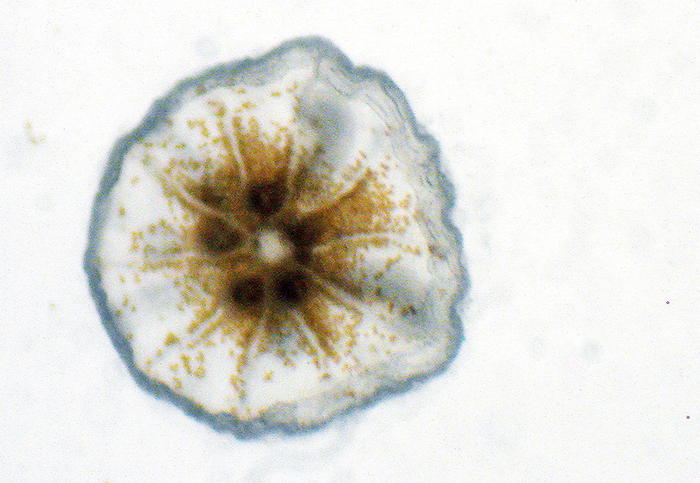
L. purpurea settled recruit with visible zooxanthellae.
Larval settlement
As previously mentioned, and described in CORAL Volume 14.1 (January/February 2017), the planula larvae of many coral species require certain chemical stimuli for settlement, and our research group has been working on identifying these for some time. Natural key stimuli are released by bacterial communities found with calcareous red algae, and we are also investigating which neurotransmitters (nerve-cell messengers) are involved in the settlement process.
As soon as a larva becomes aware of the natural key stimulus, a signal chain is set in motion that controls its attachment to the substrate and metamorphosis into a primary polyp. Because the larvae of L. purpurea depend on these specific key stimuli, they can readily be stored until settlement is triggered. If the larvae are kept in water that is as bacteria-free as possible and transferred into fresh containers at regular intervals, they can be kept in good condition for several weeks. In our experience, this storing doesn’t affect settlement behavior.
Which neurotransmitters are involved in this signal chain is still unknown. One approach to obtaining first indications is to expose the larvae to increased concentrations of any neurotransmitters that might be involved. If the tested substances play a role in settlement, then an appropriate reaction should be noticeable. Undoubtedly this will also affect other processes in the larvae, but the triggering of settlement behavior is a strong indication that the tested substance is involved in that process.
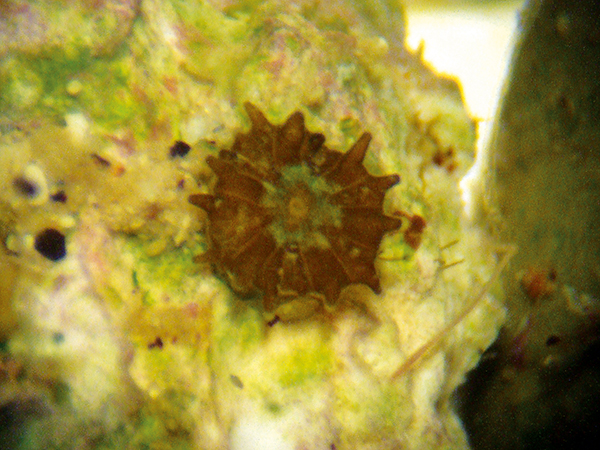
L. purpurea primary polyp that developed in the laboratory from a settled larva.
Disrupted larval development
The knowledge gained from this type of basic research may be of use in environmental protection and possibly aquaculture as well. Should a particular transmitter play a particularly important role, then similarly constructed molecules that enter the environment via wastewater or in some other way could disrupt the natural settlement process and trigger premature settlement or metamorphosis. This would inevitably lead to the death of the larvae. As most corals reproduce only once a year, an entire generation could be wiped out this way.
A prominent example of chemical pollutants that cause major problems for corals is oxybenzone. This molecule is an important component of sunscreen and is used mainly in the U.S. Oxybenzone interferes with many vital processes in corals and can, for example, cause calcification in coral larvae (Downs et al., 2016). Because calcification doesn’t normally take place in coral larvae, “switching on” the gene responsible for it causes larval death. Critical concentrations can easily be attained in bays and other areas heavily frequented by tourists, which is why Hawaii has recently banned the use of sunscreen containing oxybenzone.
For this reason, a better understanding of elementary processes such as larval settlement contributes to the protection of coral reefs. But aquaculture may also benefit from these findings. Nowadays larval settlement in other marine invertebrates, such as oysters, is sometimes triggered by neurotransmitters in commercial breeding (Coon et al., 1985, Teh et al., 2012). In this way it is possible to control the exact time of settlement as well as the settlement substrate. The larvae aren’t harmed in the process and develop normally after settlement.
If, in the near future, the reproduction of stony corals by sexual reproduction comes to play a role in restoration and/or commercial aquaculture, targeted settlement of the larvae via the use of neurotransmitters might permit use of “clean” substrates wherever possible. If the larvae are to be allowed to settle on natural biofilms, then a suitable substrate such as calcareous red algae on live rock must either be produced in aquaculture units or taken from the natural habitat. Or the desired settlement substrate must be kept in natural sea water for several weeks to develop the appropriate biofilms. These methods take a lot of time and carry the risk of introducing predators on young corals or other organisms that cause problems in the propagation of corals. Plus, the removal of settlement substrate from the wild should be avoided in the context of propagating in a manner that is as sustainable and environmentally friendly as possible.

Three-year-old aquarium-grown L. purpurea colonies spawned in 2015.
Determining threshold values for stress factors
The ability to collect larvae over a period of time allows the raising of large numbers of young corals of the same age. To obtain primary polyps, first the larvae are provided with settlement substrates, such as small pieces of calcareous red algae over a period of about 48 hours. After this period, the pieces of substrate are examined under a stereo microscope for settled and metamorphosed larvae. Colonized pieces are then glued to a PVC grid or glass tiles, and the corals are raised from there. Leptastrea purpurea thus offers us the opportunity to produce young corals of different age groups so that a direct comparison of these age groups is possible.
The effects of many stress factors on young corals are largely unknown, although their effects on adult corals have been studied and threshold values have already been established in some cases. Meaningful data can be obtained and threshold values determined through the simultaneous use of young corals of different ages. We also chose this approach in order to determine the period during which young corals are most sensitive to the ingress of sediment (Moeller et al., 2017). (More about this in a forthcoming issue of CORAL.)
Sedimentation is a major problem for coral reefs in many tropical coastal regions of the world. Often large amounts of sediment are carried onto the reefs as a result of coastal construction, and this can be very damaging to young corals even if threshold values are observed. A sedimentation event that takes place after the larvae from a mass spawning have settled could destroy an entire generation.
The work with L. purpurea makes it possible to carry out such experiments independently of the annual mass spawning. With a constant supply of larvae, experiments do not have to be planned based on a single opportunity per year, but can be performed gradually, using previously obtained results.

Three-year-old aquarium-grown L. purpurea colonies spawned in 2015.
Obtaining larvae in the aquarium
The production of L. purpurea larvae can also be carried out in the aquarium. Some of our young stock obtained in Guam in 2015 have been imported to Germany and have been living in our aquarium facility in Wilhelmshaven ever since. Although the corals grow very slowly and after just three years have reached a diameter of only 0.75–1.25 inches (2–3 cm), we were able to collect the first larvae from them in autumn 2017. The record number of larvae from our 35 or so corals stands at around 30 in a day. This number is, of course, comparatively small, but it is sufficient to continue our research into settlement behavior. In addition to the experiments with the larvae themselves, one of our goals is to grow on an F2 generation.
We will be interested to see if L. purpurea makes the grade as a study subject among other scientists and perhaps one day becomes established as a model organism for coral research. There is no doubt that this rather inconspicuous—but nonetheless highly fascinating—coral species has considerable potential. Reef aquarists may be tempted to keep and rear sexually-produced spawn from particularly colorful and attractive strains that are now starting to appear more frequently in the aquarium trade.
References
Budd, A. F., H. Fukami, N., D. Smith & N. Knowlton (2012): Taxonomic classification of the reef coral family Mussidae (Cnidaria: Anthozoa: Scleractinia). Zool .J. Linn. Soc. 166: 465–529.
Coon, S. L., D. B. Bonar & R. M. Weiner (1985): Induction of settlement and metamorphosis of the pacific oyster, Crassostrea gigas (Thunberg), by L-DOPA and catecholamines. J. Exp. Mar. Bio. Ecol. 94: 211–221.
Downs, C. A., E. Kramarsky-Winter, R. Segal, J. Fauth, S. Knutson, O. Bronstein, F. R. Ciner, R. Jeger, Y. Lichtenfeld, C. M. Woodley, P. Pennington, K. Cadenas, A. Kushmaro & Y. Loya (2016): Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and its Environmental Contamination in Hawaii and the U. S. Virgin Islands. Arch. Environ. Contam. Toxicol. 70: 265–288.
Moeller, M., S. Nietzer, T. Schils & P. J. Schupp (2017): Low sediment loads affect survival of coral recruits: The first weeks are crucial. Coral Reefs 36: 39–49.
Nietzer, S. (2016): Korallenfarm 2.0—geschlechtliche Nachzucht. KORALLE 100, 17 (4): 32–41.
Nietzer, S. & M. Möller (2017): Guam—Eine abgelegene Pazifikinsel lädt zum Entdecken ein. KORALLE 104, 18 (2): 50–57.
Rohleder, P.-G. (2015): Korallen aus der Gattung Leptastrea. Der Meerwasseraquarianer 4/2015: 46–53.
Teh, C. P., Y. Zulfigar & S. H. Tan (2012): Epinephrine and l-DOPA promote larval settlement and metamorphosis of the tropical oyster, Crassostrea iredalei (Faustino 1932): An oyster hatchery perspective. Aquaculture 338–341: 260–263.
Veron, J. E. N. (2000): Corals of the World. Australia Institute of Marine Science (AIMS), Queensland, Australia.

Jason Fox “John Deere” Leptastrea. Image: May Fox
Read More
CORAL Editorial: The Next Threshold in Reefkeeping
Author
Samuel Nietzer is a Ph.D. candidate and researcher at Carl von Ossietzky Universität Oldenburg, Wilhelmshaven, Germany. He is a frequent contributor to CORAL and its sister publication in Germany, KORALLE.





